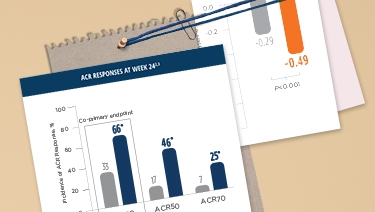
rapid, sustained, consistent clinical response
ACR20 clinical response was observed in MTX-IR and TNF-IR patients as early as 2 weeks in MOBILITY and as quickly as 4 weeks in TARGET, respectively, after the first dose. 1
*P<0.0001; †P<0.01.
‡DMARD(s) in TARGET and MOBILITY include MTX, sulfasalazine, leflunomide, and/or hydroxychloroquine.
KEVZARA provided patients with uncontrolled RA
fast, lasting clinical improvement1
§P<0.0001, P value is for descriptive purposes only and is not adjusted for multiplicity.
||P<0.0001.
MTX-IR=methotrexate inadequate response; TNF-IR=tumor necrosis factor inhibitor inadequate response or intolerant; ACR20=American College of Rheumatology 20% improvement criteria; MTX=methotrexate; q2w=once every 2 weeks; DMARDs=disease-modifying antirheumatic drugs.
50%
Week 16: KEVZARA 200 mg + MTX: 57% (229/399) vs placebo + MTX: 42% (169/398);
Week 52: KEVZARA 200 mg + MTX: 48% (190/399) vs placebo + MTX: 26% (104/398)
*Minimal clinically important difference. HAQ-DI change from baseline ≥0.3 units.
HAQ-DI=Health Assessment Questionnaire-Disability Index; MTX-IR=methotrexate inadequate response; TNF-IR=tumor necrosis factor inhibitor inadequate response or intolerant; ITT=intent to treat; MTX=methotrexate; q2w=once every 2 weeks; DMARDs=disease-modifying antirheumatic drugs.
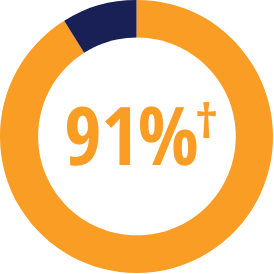
RADIOGRAPHIC PROGRESSION
56% OF MTX-IR PATIENTS HAD NO RADIOGRAPHIC PROGRESSION WITH KEVZARA
AT 52 WEEKS vs 39% OF PATIENTS TREATED WITH PLACEBO2*
greater inhibition of joint damage progression
with KEVZARA 200 mg + MTX vs placebo + MTX2
*Defined as mean change from baseline in total Sharp score of ≤0.
†Based on post hoc comparisons of mean change in mTSS per treatment group. Week 52 analysis employs linear extrapolation method to impute missing or post rescue data.2
MTX-IR=methotrexate inadequate response; mTSS=modified total Sharp score; MTX=methotrexate.
INTERPRET WITH CAUTION
ACR20=American College of Rheumatology 20% improvement criteria; DMARDs=disease-modifying antirheumatic drugs.
INTERPRET WITH CAUTION
Data in patients who received initial treatment with KEVZARA compared with those who initially received placebo.
MOBILITY EXTEND
This analysis has not been performed for the TNF-IR population.
*Mean HAQ-DI scores were calculated based on observed cases, without imputation of missing data.
HAQ-DI=Health Assessment Questionnaire-Disability Index; SE=standard error; MTX=methotrexate; q2w=once every 2 weeks; TNF-IR=tumor necrosis factor inhibitor inadequate response or intolerant.
HAQ-DI was measured in patients who
continued treatment with KEVZARA for up to 5 years1,5
INTERPRET WITH CAUTION
OVER 5 YEARS
MOBILITY EXTEND
*Radiographic progression was assessed by change from baseline in mTSS, reported using a post hoc integrated analysis of 4 separate reading campaigns. Change from baseline in mTSS was recorded in 1 campaign during the randomized controlled phase and 3 campaigns during the OLE. A post hoc integrated analysis was conducted to analyze radiographic progression based on all available campaign data.5
mTSS=modified total Sharp score; SE=standard error; MTX=methotrexate.
Inhibition of structural progression was measured in patients who
continued treatment with KEVZARA for up to 5 years5



